Maximum Likelihood Estimation (MLE)
Maximum Likelihood Estimation (MLE) is an objective technique for selecting parameter values in a model by maximizing the likelihood of observing the collected data under the chosen model. In quantal toxicity tests, the number of test organisms affected at a given concentration follows a binomial distribution. The parameters of this binomial distribution are typically related to concentration through a normal distribution or logistic function. The Probit Method is particularly suitable for handling binomial response data (e.g., survival/death). A critical requirement is that the toxicity data must include at least two partial effect levels between 0% and 100% effect.
In probit regression, MLE is considered equivalent to iterative least squares methods (Christoph and Moore, 1975). In other words, MLE is an iterative technique for estimating values such as LC50, EC50, and LD50, along with their confidence intervals. It is mathematically more elegant, focusing on two parameters in probit regression analysis: the slope and the intercept.
Advantages of Maximum Likelihood Estimation (MLE)
- Precision: Provides reliable estimates even with small sample sizes compared to least squares methods.
- Handling Control Effects: Can incorporate control effects as separate parameters in complex models.
- Confidence Intervals: Calculates asymptotic variance using the Hessian matrix (inverse of the second derivative), enabling interval estimation via Wald or likelihood ratio tests.
Disadvantages of Maximum Likelihood Estimation (MLE)
- Computational Complexity: May fail to converge with small datasets.
- Assumption Dependency: If the data does not follow a log-normal distribution, bias may occur. In such cases, a logit model (logistic regression) may be used as an alternative.
Analysis Results of ToxGenie’s Maximum Likelihood Estimation (MLE) of the Probit Method
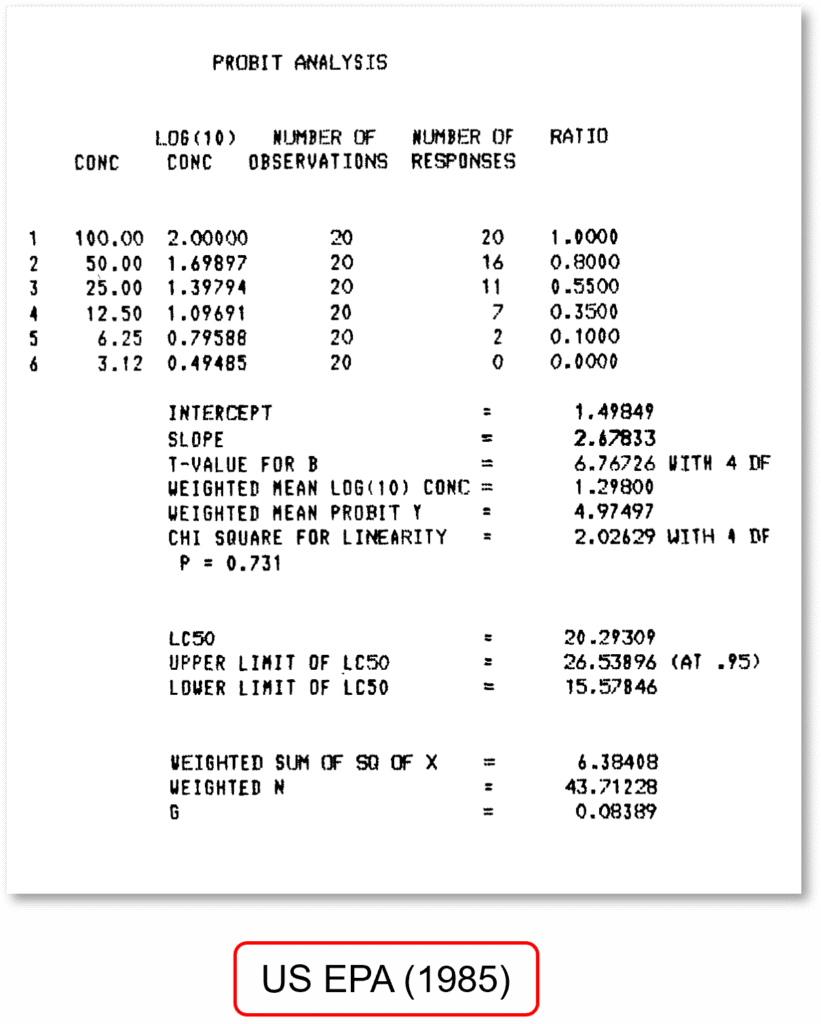
To compare ToxGenie’s analysis results, the Maximum Likelihood Estimation of the Probit Method was applied using the same data as in the US EPA Report (EPA 600/4-85-013, 3rd edition, 1985). Due to differences in the number of decimal places used in mathematical calculations, rounding criteria, and the use of direct calculations instead of pre-calculated empirical probit percent mortality relationship tables, the results are not identical to those of the US EPA analysis. However, they demonstrate high accuracy. For point estimation analysis, ToxGenie recommends the Maximum Likelihood Estimation of the Probit Method for the US EPA data. If the user prefers a different method, ToxGenie can also perform the analysis using the user-specified method.
Start Your Toxicology Data Statistical Analysis with ToxGenie
Understanding quantal and quantitative data is the first step to robust toxicology research. ToxGenie offers an intuitive platform for analyzing both data types, suitable for beginners and experts alike. Curious? Start with a 30-day free trial and revolutionize your toxicity data statistical analysis today!