Effortless Toxicity Data Analysis with ToxGenie
Focus on your research with user-friendly statistical analysis software for toxicity data
No credit card needed!
How ToxGenie Was Born...
Built from 30 Years Solving Real Toxicity Data Statistical Analysis Challenges
For over 30 years as an ecotoxicologist, I’ve conducted numerous GLP (Good Laboratory Practice) studies and ERA (Environmental Risk Assessment) research, I’ve now reached a point where I can develop software for analyzing toxicity data. However, I too spent countless nights on statistical analysis to process the results from toxicity tests and field studies.
I often found errors in the statistical analyses in research papers and reports from my junior colleagues. When I asked them if statistical analysis was difficult, many more of them said it was a headache than those who said it was easy. This made me realize that junior scientists are still facing the same struggles I did.
ToxGenie automates tasks like Point Estimation and Hypothesis Tests, delivering regulatory-compliant results in minutes. It streamlines acute toxicity tests and multi-endpoint studies, saving time and ensuring accuracy for toxicologists.
Designed for ease, ToxGenie’s intuitive interface requires no statistical expertise and aligns with OECD and US EPA standards. It saves about 70% time on toxicity data statistical analysis, empowering researchers to focus on on your research, not time-consuming data analysis, as a reliable partner. “Click here for a more detailed story.”
Struggling with Toxicity Data Analysis?
Toxicological researchers face time-consuming analyses, complex statistical methods, and the risk of unreliable results.
ToxGenie eliminates these challenges with an intuitive, end-to-end solution.
Time-Consuming Analysis
Manual data processing and method selection delay your research.
Complex Statistics
Choosing the right statistical method requires expertise and effort.
Risk of Errors
Incorrect analyses can undermine your research’s credibility.
Meet ToxGenie
Your Toxicology and Ecotoxicology Research Partner
Unlike general-purpose statistical tools, ToxGenie is designed specifically for toxicology and environmental toxicology.
With its user-friendly GUI, specialized dose-response analysis, three-step analysis process, and automated reporting,
ToxGenie saves time and delivers reliable, regulatory-compliant results.
ToxGenie Workflow
Example Workflow: Determining Acute Definitive Test Concentrations in 3 Simple Steps

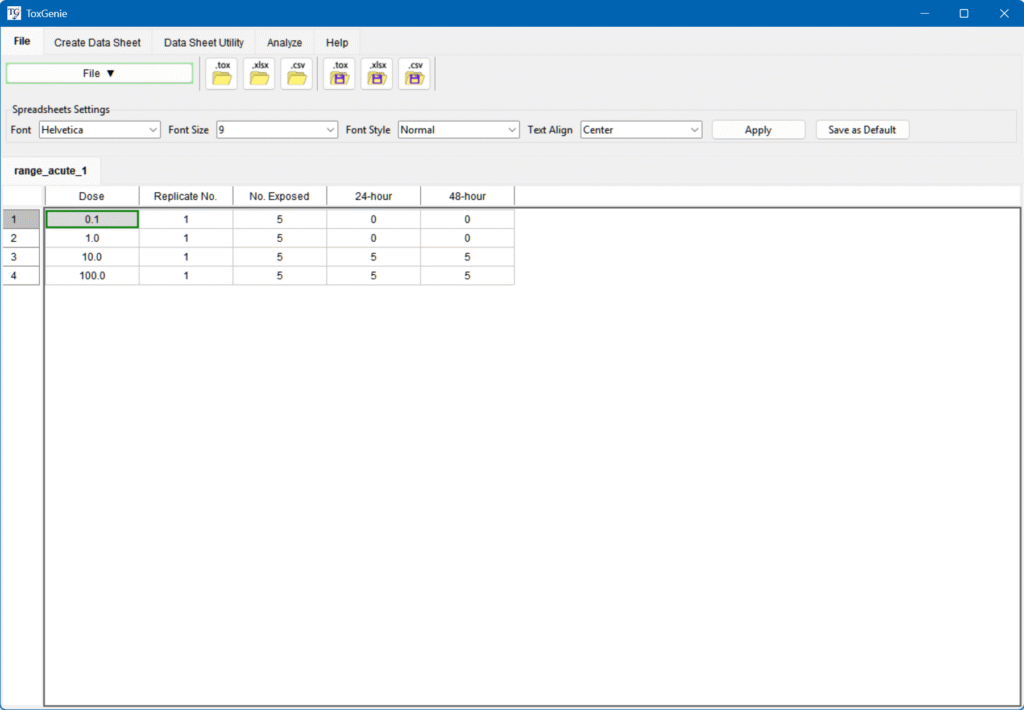
Enter Your Data
- Using the Create Data Sheet menu to input data directly.
- Modifying an Excel file in the Sample Data folder, saving it as .xlsx or .csv.
- Importing it via the File menu.

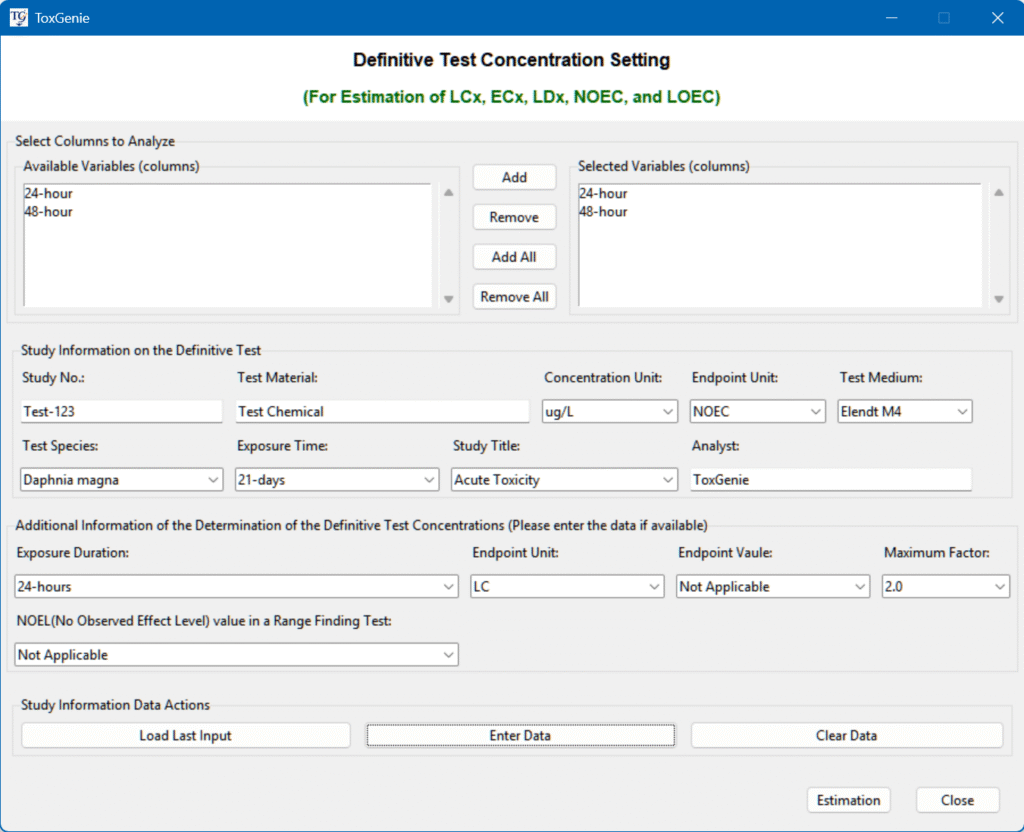
Run Estimation
- Select Analyze tab.
- Select Definitive Study Concentration Setting in the drop-down menu.
- Enter or select all information related to the test.
- Select Acute Toxicity in the Study Title.
- Click estimation button.

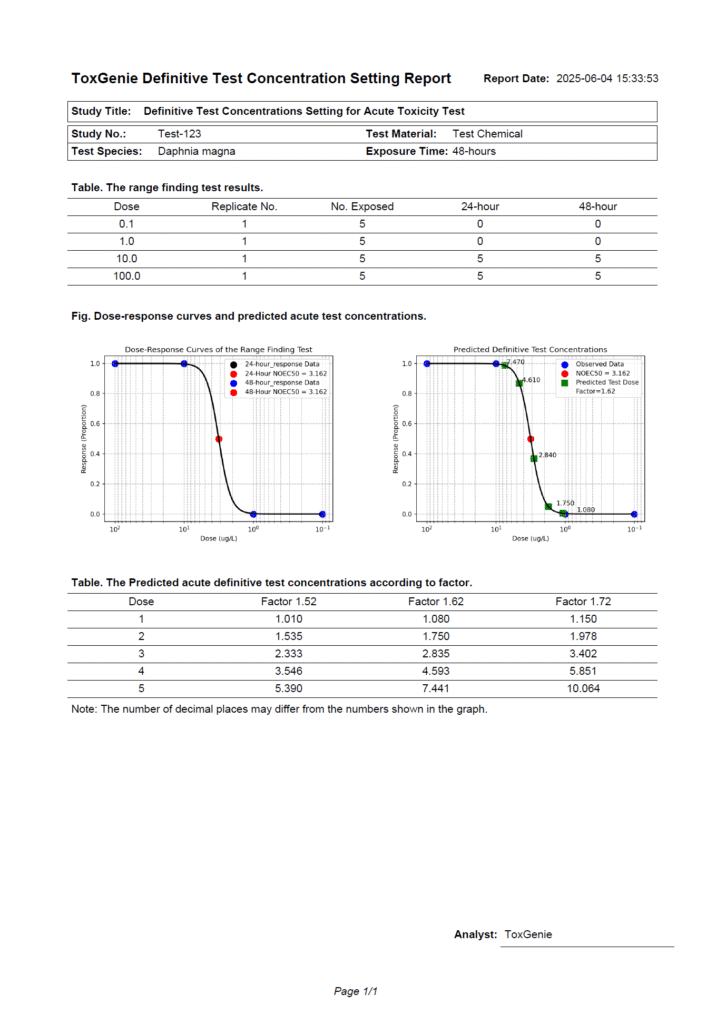
Generate Report Automatically
- Print or Save As the PDF report.
Why Choose ToxGenie?
Save Time
Reduce analysis time by up to 70% with automated workflows and a one-stop solution for statistical analysis of toxicological data.
Enhance Reliability
Ensure accurate results with validated algorithms and toxicological interpretations.
User-Friendly
Designed for researchers, not statisticians, with an intuitive interface.
Trusted by Experts
Tested and used by leading universities, GLP facilities, and toxicology labs, and fully compliant with international standards.
Powerful Features for Toxicity Data Statistical Analysis
ToxGenie provides specialized tools to support a wide range of toxicological studies.
Determination of Definitive Test Concentrations
Automatically recommend optimal concentrations for acute and chronic toxicity tests using range finding test data.
LC50, EC50, LD50 Calculation
Calculate LC50, EC50, LD50 with 95% confidence intervals for acute and chronic toxicity tests.
IC25, IC50 Analysis
Estimate IC25 and IC50 with 95% confidence intervals for ecotoxicity and cytotoxicity tests by automatically selecting the best-fit model.
Estimation of NOEC and LOEC for a Single Observation
Provide a comprehensive analysis for a single endpoint, determining the NOEC and LOEC for both acute and chronic toxicity studies.
Estimation of NOEC and LOEC for Multiple Observations
Provides comprehensive one-stop analysis for multiple endpoints across three or more test groups in studies such as Daphnia Reproduction and Fish ELS tests.
Algae and Cyanobacteria, Growth Inhibition Test Analysis
One-stop analysis for test validity, EC10, EC20, EC50, NOEC, and LOEC for both average growth rate and yield.
Two-Group Comparisons
Automatically compares one or more endpoints between two groups in Limit Test.
Regulatory Compliance
Align with OECD, US EPA, EU-REACH, and K-REACH standards for global acceptance.
Intuitive GUI that requires no user manual
No statistical expertise is required, and it is so easy to use that a user manual is not needed. You can complete your analysis with just a few clicks.